Introduction
The word cancer still remains the most dreaded diagnosis in any medical setting. People often mistake this as a distant entity, something that happens to “other people”. The data on its prevalence suggests otherwise, cancer afflicts almost families.
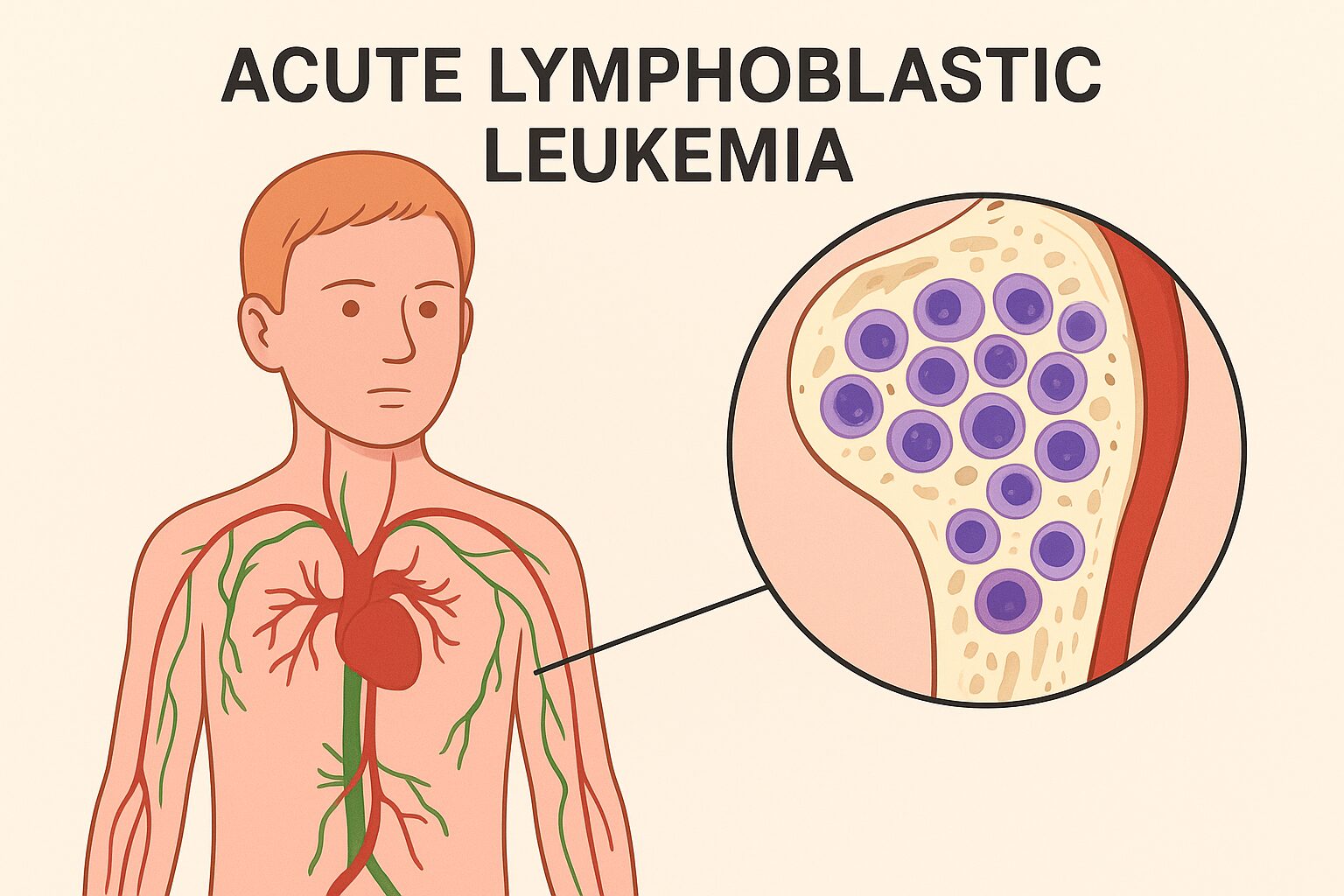
Prevalence of blood cancers. However, these blood cancers have several varying subtypes, each affecting a different demographic, sharing varied prognostic factors and having distinct etiologies. Acute lymphoblastic leukaemia (ALL) is one of the most common blood cancers occurring in the pediatric age group.It accounts for 25% of all pediatric cancers. Although it does sounds like complex medical jargon, it is imperative to understand it in simpler language to make us better equipped with knowledge, crucial to support those to face it.
What is ALL?
Leukaemia itself means a haematological malignancy (blood cancer). Blood is made up of various cells, especially the red blood cells, the white blood cells and the platelets. The bone marrow is a crucial site for the balanced production of these blood cells. In ALL, the maturation of these cells is hampered. So, immature cells called as lymphoblasts proliferate uncontrollably. The overproduction disrupts the balance. This disease can be related to a software error in the genetic code of the human machinery that produces mature lymphocytes. The immature lymphocytes, instead of maturing, continue to multiply. These immature cells do not function similarly to the mature cells, resulting in a deficiency of RBC, WBC and Platelets.
Insufficient Red Blood Cells result in anaemia, fatigue and pallor. The lack of platelets results in bleeding and bruising, and a decrease in WBC results in recurrent infections.
ALL is further classified into B-cell ALL and T-cell ALL. B-cell ALL is more common and it accounts for 80-85 % of the cases. Molecular subtyping has revealed genetic abnormalities like Philadelphia chromosomes (BCR-ABL1 fusion), MLL rearrangements, or ETV6-RUNX1 translocation. The understanding of the underlying genetic abnormality guides treatment decisions and prognosis. For instance, children with ETV6-RUNX1 often have excellent outcomes, while targeted therapy is warranted in those with BCR-ABL1 mutation (tyrosine-kinase inhibitors).
Risk Factors
ALL is known to afflict 3-4 per lakh children annually. The most common age group affected is 2-9 years. Adults can also get ALL. ALL has a bad prognosis if it affects children <1 year and >10 years. Genetic syndromes like Down Syndrome or Li-Fraumeni are risk factors for ALL. Others include, previous history of chemotherapy or radiation, and a family history of leukaemia (rare). The cause is idiopathic for most of the patients.
Symptoms
The clinical presentation of ALL is due to a deficiency of mature blood cells. There can be pallor and fatigue due to decreased haemoglobin. There is an increased risk of infections due to WBC deficiency. The decrease in platelets leads to bleeding tendencies like petechiae and purpura. Due to the inability of the bone marrow to synthesise mature cells, extramedullary hematopoiesis occurs, resulting in hepatosplenomegaly, bone pain, sternal tenderness and lymphadenopathy. ALL is characterised by the involvement of the CNS, testis, spleen and lymph nodes. This makes it distinct from Acute Myeloid Leukaemia. T-ALL can include involvement of the mediastinum as well.
An early sign of the disease can be refusal to walk or play by the child due to bone pain. The symptoms of ALL can be progressive and persistent, unlike simple viral fevers that resolve in days.
Diagnosis
The complete blood count raises suspicion, but the diagnosis needs to be confirmed by a bone marrow aspiration or biopsy. IT shows more than 20 % lymphoblasts. Flow cytometry can identify the cell lineage (B-Cell or T-Cell). The cytogenetics and molecular subtyping can further reveal prognostic mutations.
ALL is one of the most studied cancers. The risk stratification is precisely done based on the minimal residual disease monitoring (MRD). It informs us that even if a small number of cancer cells persists after treatment, making it a powerful predictor of the relapse.
Treatment
The treatment of ALL is long and well-structured.
It includes three phases: The Induction phase, the consolidation/intensification phase, and the maintenance therapy. The induction phase is carried out in 2 cycles. The goal is to achieve remission from the disease. The regimen includes steroids, vincristine, daunorubicin and cyclophosphamide. After the induction phase, the bone marrow study is carried out. The MRD status is checked. If positive, then 2 cycles of chemotherapy are done. If MRD is still positive, then a bone marrow transplant is warranted. If the MRD status is negative, then the consolidation phase is done, which includes two cycles of chemotherapy. This is followed by the maintenance phase, which includes methotrexate and 6-mercaptopurine for 2-3 years.
The newer advances in treatment include targeted therapy (tyrosine kinase inhibitors like imatinib for Philadelphia-positive chromosomes), Immunotherapy ( Blinatumomab: bispecific T-cell engager; CAR-T cell therapy) and supportive care (antimicrobials, blood transfusion, nutritional support and psychological counselling).
The Human Outlook
The treatment of ALL is long, cumbersome and entails several difficulties for the parents and the child. It brings financial strain, emotional exhaustion and social stigma. The sibling may feel neglected. This is where community support plays a role. The schools can offer flexible scheduling, and NGO can offer funds. Support groups can be created to share experiences to lighten the family burden and serve as a crucial information source.
Surviving ALL
The story of ALL does not end with remission. Children can suffer from late effects like growth and hormonal imbalance, learning difficulties, and fertility issues. There can also be an increased risk of secondary cancers.
Conclusion
ALL is far from just a disease in a medical textbook. It is a reality for thousands of families. The progress in science has improved the survival rate. The increased awareness has fostered community strength, enabling families to endure the long, difficult road.
ALL is a disease that requires early recognition, aggressive treatment and endless support. The treatment of ALL is not just about saving life but giving the child his laughter, dreams and future back.
References
1. Pui C-H, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371(9617):1030–1043.
2. Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–1552.
3. National Cancer Institute (NCI). Childhood Acute Lymphoblastic Leukemia Treatment (PDQ®) – Patient Version. https://www.cancer.gov
4. World Health Organization (WHO). Childhood cancer fact sheet. https://www.who.int
5. Indian Council of Medical Research (ICMR). National Cancer Registry Programme Report 2020