Unlocking the Power of Regeneration – The Science of Healing from Within

Imagine if the body could heal itself from within—a heart repairing its own damage, lost tissue growing back as if it were never gone, or even entire organs regenerating using nothing but your own cells. This is not just a dream of the future; it’s the remarkable promise of modern science, bringing us closer to a world where the body holds the key to its own healing.
For thousands of years, the approach in medicine has been managing diseases and their symptoms. Nature, however, reveals a different picture: one that focuses on healing and renewal. Like how starfish can regrow a limb or a salamander can self-heal its spinal cord. These are not miracles; rather, they serve as a reminder that the machinery to self-repair already exists within living beings. What would happen if humans could access this power?
But how humans can tap into the potential? This is where the groundbreaking field of regenerative medicine comes in—to Repair, Replace, and even Regenerate damaged tissues and organs using Stem Cells, Gene Editing, and Tissue Engineering.

It all started back in 1868, when Ernst Neumann identified the regenerative capacity of bone marrow. The first concept of stem cell was described by Alexander Maksimov in 1908. Then in 1956, Dr. E. Donnall Thomas conducted the first bone marrow transplant, demonstrating the body’s partnership in its healing. A big breakthrough came in 1998, when James Thomson isolated Human Embryonic Stem Cells and a second breakthrough in 2006, Once adult cells were able to revert back to a stem cell state Shinya Yamanaka’s vision came to life through Induced Pluripotent Stem Cells (iPSCs).
Now the future of healthcare is ever changing. Regenerative efforts such as growing organs in labs, CRISPR’s gene editing wonders, and 3D bioprinting of tissue means organ transplants may just be a relic in the not-so-distant future.
With millions suffering from Organ Failure, Neurodegenerative Diseases, and Aging-Related conditions, Regenerative Medicine offers a groundbreaking solution—the ability to Heal from within, not just treat symptoms.
The future of medicine is here. Are we ready to embrace it?
Stem Cells in Regenerative Medicine Classification
Embryonic stem cells (ESCs)
Source: Inner cell mass of blastocysts during early embryonic development.
Pluripotent: Able to become any number of various types of cells.
Applications in neurological repair, cardiac regeneration, organoid models
Limitations: Ethical concerns; higher risk of tumorigenesis.
Adult Stem Cells (ASCs)
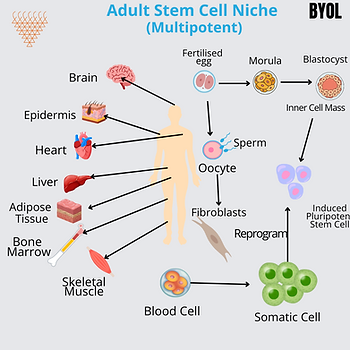
- Present in bone marrow, adipose tissue and organ-specific niches.
- Multipotent, or capable of differentiating along a certain cell line (hematopoietic – blood formation and mesenchymal).
- Used in bone marrow transplants, cardiovascular therapy, and cartilage repair.
Induced Pluripotent Stem Cells (iPSCs)
- Reprogrammed from somatic cells using transcription factors (e.g., OCT4, SOX2, KLF4, c-MYC).
- Overcomes immune rejection and ethical concerns of ESCs.
- Applied in disease modeling, drug discovery, and patient-specific therapies.
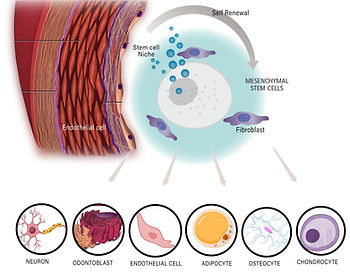
Mesenchymal Stem Cells (MSCs)
- Found in bone marrow, adipose tissue, umbilical cord, and synovial fluid.
- Multipotent: Can differentiate into bone, cartilage, muscle, and adipose tissues.
- Exhibits anti-inflammatory and immunomodulatory properties.
- Used in orthopedic, cardiovascular, and autoimmune disease treatments.
Mechanisms of Regeneration:
Stem Cell Differentiation and Tissue Repair
- Stem cells differentiate into functional cell types under the influence of growth factors (e.g., BMP, VEGF, TGF-β), extracellular matrix (ECM) signals, and epigenetic modifications.
Paracrine Signaling and Immunomodulation
Stem cells secrete exosomes, cytokines, and microRNAs (miRNAs) that:
- Stimulate angiogenesis (blood vessel formation).
- Reduce inflammation and modulate immune responses.
- Promote tissue regeneration without direct cell transplantation.
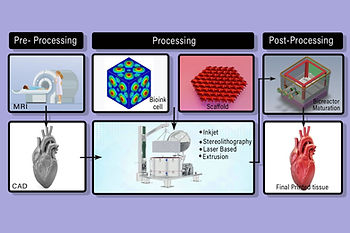
Bioprinting and Tissue Engineering:
3D Bioprinting mechanism
- Uses bio- inks containing live cells and growth factors.
- Microfluidics and bioreactors regulate oxygen, nutrients and mechanical stimuli for tissue maturation.
Scaffold-Guided Regeneration
• Biodegradable scaffolds ( eg- Collagen, Hydroxyapatite, Polylactic acid) provide a framework for cell attachment and proliferation.
Clinical Applications Of Regenerative Medicine and Stem Cells
Neural Regeneration and synaptic Repair Mechanism
- Axonal guidance molecules (Netrins, Semaphorins, Ephrins)Directing neuron Growth.
- BDNF( Brain – Derived Neurotrophic Factors) and NGF( Nerve Growth Factor) promoting synapse formation.
- Glial Neurogenesis for stroke and spinal cord injury recovery.
Cardiovascular Regeneration:
Endothelial Progenitor Cells (EPCs)
- Restore blood vessels by activating VEGF (Vascular Endothelial Growth Factors) pathway.
Angiogenesis Promotion
- Secretion of Angiopoietins and FGS( Fibroblast Growth Factors) to stimulate new blood vessel in ischemic issues.
Orthopedic and Musculoskeletal Repair
- Bone Defects: MSCs with calcium phosphate scaffolds promote osteogenesis.
- Cartilage Repair: Autologous chondrocyte implantation (ACI).
- Tendon/Ligament Injuries: Stem-cell-loaded biopolymer scaffolds aid tissue regeneration.
Organ Regeneration and Transplantation
- Liver Regeneration: Hepatic stem cells replace damaged liver cells by activating Wnt/ Beta- catenin and Notch signaling.
- Kidney regeneration: Podocytes stem cells improve kidney filtration in chronic kidney disease.
- Lung Tissue Engineering: Decellularized lung scaffolds populated with progenitor cells.
Endocrine and Metabolic Disorders
- Diabetes Mellitus: β-cell replacement therapy using iPSC-derived insulin-producing cells.
- Thyroid Disorders: Stem-cell-based thyroid tissue regeneration.
Cancer Therapy and Stem cells
- CART- T Cell Therapy: Genetically engineered T cells attack cancer by expressing Chimeric antigen receptors (CARs).
- Tumor- Targeting MSCs: Modified stem cells delivery Oncotoxic drugs directly to cancerous tissues.
Challenges and Ethical Considerations
Despite Advancements, Regenerative Medicine Faces Scientific, Regulatory, and Ethical Barriers:
- Immune Rejection & Tumorigenesis – iPSC-derived cells risk immune incompatibility or tumor formation.
- Ethical Concerns – ESC research remains controversial due to embryo destruction.
- Standardization & Quality Control – Clinical translation requires Good Manufacturing Practices (GMP).
- Cost & Accessibility – Stem cell therapies are expensive and not widely available.
Future Directions and Emerging Technologies:
Gene therapy and CRISPR based Regeneration –
CRISPR- Cas9 Gene editing
- Correcting defective genes in genetic disorders by targeting specific DNA sequences.
- Used to correct mutation in sickle cell anemia, Duchenne muscular dystrophy and cystic fibrosis.
Genetic infusion system
- Viral vectors – ( Adenovirus, lentivirus) for inserting Regenerative genes into stem cells.
- Non viral vectors – ( Lipid nanoparticles, Electroporation) for safer gene transfer.
Organoids and Lab-Grown Tissues
- Miniature organ structures used for drug screening and transplantation research.
- Advances in kidney, liver, and brain organoid technology are revolutionizing preclinical testing.
AI and Machine Learning in Stem Cell Research
- AI models predict stem cell differentiation patterns.
- Machine learning optimizes personalized cell-based therapies.
Personalized Regenerative Medicine
- Patient-derived iPSCs minimize immune rejection.
- Customized biomaterials enhance tissue integration.
Conclusion
Regenerative medicine is a transformative field that integrates biotechnology, stem cell biology, tissue engineering, and gene therapy to restore or replace damaged tissues and organs. At its core, it relies on technical mechanisms that drive cellular regeneration, differentiation, and integration within the body. Stem cells play a crucial role in this process, utilizing various molecular and biomechanical pathways to repair and regenerate tissues.
References
1. Mason C., Dunnill P. A brief definition of regenerative medicine. Regenerative Medicine. 2008;3(1):1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
2. Fortier L. A. Stem cells: classifications, controversies, and clinical applications. Veterinary Surgery. 2005;34(5):415–423. doi: 10.1111/j.1532-950x.2005.00063.x. [DOI] [PubMed] [Google Scholar]
3. Shroff G., Gupta R. Human embryonic stem cells in the treatment of patients with spinal cord injury. Annals of Neurosciences. 2015;22(4):208–216. doi: 10.5214/ans.0972.7531.220404. [DOI] [PMC free article] [PubMed] [Google Scholar]
4. Banas A, Teratani T, Yamamoto Y, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology 2007;46:219-28. 10.1002/hep.21704 [DOI] [PubMed] [Google Scholar]
5. Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol 2016;2016. [DOI] [PMC free article] [PubMed]
6. Levenberg S, Huang NF, Lavik E, et al. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A 2003;100:12741-6. 10.1073/pnas.1735463100 [DOI] [PMC free article] [PubMed] [Google Scholar]