Introduction
In susceptible individuals, progressive neuronal degeneration is a hallmark of neurodegenerative diseases (NDs). Neuronal malfunction and the eventual loss of neurons have been caused by several important physiological processes such as oxidative stress, proteotoxic stress, neuroinflammation, and apoptosis. Bothering public health, neurodegenerative diseases carry a heavy burden on the healthcare systems worldwide due to immunological activation within the central nervous system (CNS). Of all NDs, Alzheimer’s (AD), Parkinson’s (PD), Huntington’s (HD), and Amyotrophic Lateral Sclerosis (ALS) are recognized as the four major diseases. Among the other strong factors related to these diseases are environmental factors, faults in immunity, increment in age, and also the genetic constitution of the affected person. Aging causes the primary contribution to these diseases due to the fact that most NDs manifest themselves in age groups of above 50 years. Aging could also be expedited through oxidative stress and mutations in mitochondrial DNA, among other causes. More than 58 million people in the world are diagnosed with AD, with the frequency of cases slowly increasing due to variables involving longer average life span along with inherited and environmental factors. Deaths due to this disease are expected to be around 152 million by the year 2050. This will become an increasing weight on the world economy.
Nanomedicine, a huge interdisciplinary area that combines nanotechnology with medicine, uses nanoparticles (NPs) at a nanoscale for the targeted delivery of therapeutic medicines, phytoconstituents, and biomolecules to specific areas of the anatomy within the body, including the brain. Nanotechnology, all of which is known, is being assiduously researched with the aim of discovering possible cures for NDs. NPs have recorded remarkable advances in enhancing the delivery of various active molecules for diagnosis and therapeutic strategies for diseases. This is mostly attributed to their vast assortment of chemical properties and the ability to chemically modify them to control enhancement of some critical characteristics. They have the ability to encapsulate molecules with a varied array of chemical compositions and provide good delivery mechanisms and protection to bioactive compounds. More recently, it has been found that NPs can help introduce molecules into tissues that are usually considered difficult to access, such as the brain. Different kinds of NPs have been shown to deliver drugs successfully into neural cells in neurodegeneration-related therapies. These include metal NPs, polymeric NPs, nanoparticle conjugates, solid lipid carriers, lipid/lipoprotein-based NPs, liposomes, magnetic NPs, dendrimers, inorganic NPs, nanocomposites, nano-emulsions, and antibody-tethered NPs.
Obstacles to CNS delivery
Drug delivery to brain tissues is the most precarious with respect to CNS therapy. It is estimated that due to delivery hindrances, nearly 98% of micro-drug molecules and 100% of proteins and nucleic acid therapies will ultimately be rejected from the CNS. Failure in therapy is because of the innate physiochemical characteristics of drugs that cause failure across blood-brain and blood-cerebrospinal fluid barriers and their dilution effects in systemic diffusion.
Blood-brain barrier (BBB)
The BBB accounts for the various reasons for most drug molecules being able to permeate various body tissues but with great difficulty in crossing into the brain. Experimental evidence supported the idea of an actual physical barrier around the boundary that separates circulating blood from the CNS. There is some evidence that the growing mechanical barrier is tightly associated with connections between the microvasculature and the endothelial cells of the brain. Proteins from claudin and occludin, situated within these tight junctions, create a strong barrier to the passage of any substance between blood and brain. A therapeutic moiety has to possess none of the following characteristics in order to cross the BBB: more than 10 hydrogen-bonded donors or acceptors, a molecular weight greater than 500 Daltons, similarity to the BBB enzyme system and BBB output system, and high binding to plasma proteins. Therefore, avoidance of BBB should be prioritized in the design and development of CNS delivery systems.
Blood cerebrospinal fluid barrier (BCSF)
Commonly referred to as the BCSF, the physiological surface separating CSF from systemic circulation functions to exclude harmful substances from the CNS just like the BBB. Certain molecules are permitted to cross the BCSF, working as a selective barrier in the formation of CSF. The BCSF acts as a secondary barrier for drug distribution, which is one thousand times smaller in surface area than the BBB.
Nanotechnology-based drug delivery strategies
a) Lipid-based nanoparticles (LNPs)
LNPs have reached the advanced age of controlled delivery of drugs to the brain. The inherent lipidic nature of these entities resembles the lipidic composition of the BBB to facilitate their transcellular passage into the brain. LPNs are mainly composed of phospholipids, fatty acids, monoglycerides, triglycerides, fatty alcohols, and waxes. The three major classes of LNPs, which are liposomes, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs), are well accepted for research and practical use, especially in CNS diseases.
b) Polymer-based NPs
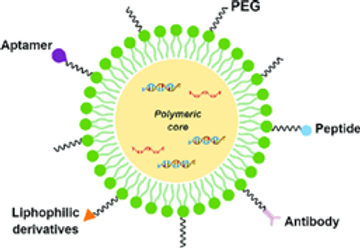
Polymeric NPs are being used for the delivery of a variety of drugs due to their long shelf life, improved biocompatibility, and biodegradability, which allow for a controlled drug release at a particular site. NPs are mostly prepared from synthetic polymers such as polylactic acid, polyglycolic acid, and polyacrylates, while natural polymers like chitosan, alginate, and albumin are being used as well.
c) Nanoscale gels
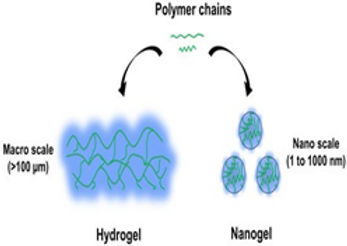
Among the advances that have afforded nanogels a status for emerging as a promising platform for the targeted delivery of drugs is their colloidal stability, as well as their very high drug loading capacity, core-shell architecture, excellent permeation characteristics, and the ability to be responsive to stimulants in the environment. Current advances in the formulation of novel nano-gel technologies, such as surface modification of receptors, ligands, and magnetic structures, are enabling various nano-gel-based approaches to be conceived and implemented in the crossing of the BBB’s defenses against targeted medications. Such technologies are now being used in the design and formation of nano-vehicles for different neuroprotective drugs, for instance, nucleic acids, peptides, nerve growth factors, and free radical scavengers. Present-day characterization of nano-gel drug delivery methods is in their early stages of development. More profound investigation over nano-gel systems should, however, precede widespread application of such treatments in the clinic for several ailments, including preclinical and clinical trial investigation.
d) Dendrimers
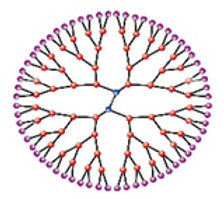
The smallest, most specifically defined nanofabrication, dendrimers, are used for the treatment of NDs in biomedical applications. The size, core-shell shape, and surface functional groups can be changed to create a whole continuum of nano-carriers that deliver drugs and genes into the brain. Dendrimers have strong anti-amyloidogenic properties, thus creating a wider avenue for possible therapeutic applications for prion disease, AD, and PD therapy.
e) Nanocomposites
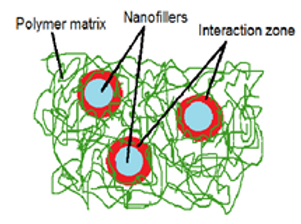
Composite is the substance formed of property combinations of various materials. Broadly, such a structure contains a matrix and probably a reinforcing phase. One-dimensional objects include tubes and threads; two-dimensional objects include layered materials such as clay, and three-dimensional objects include spheres. In line with our increasing understanding of the pathogenic mechanisms involved in AD/PD, new molecular targets that might help in alleviating neurodegeneration were discovered. Different forms of treatment have been proposed for cognitive deficits, avoiding oxidative stress, and pathologic protein misfolding and relative aggregation by neuroprotective proteins. Proteases such as Hsp70 and Hsp27, which degrade harmful protein aggregates, and pro-survival chaperone proteins like these may offer exciting new possible avenues for these protein-based treatments.
f) Metal-based NPs
i) Gold NPs (AuNPs)
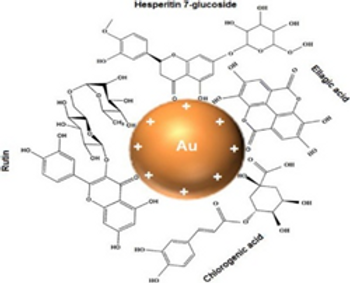
The usefulness of AuNPs in various applications has made them a popular subject of research. It is easy to fabricate gold into nanometer-scaled materials due to its unique properties. Applications include radiation therapy, thermal ablation, drug delivery, and medical diagnosis, among others. Given the difficulties of delivering therapeutic drugs through the BBB in NDs, some studies are being evaluated using AuNPs in BBB models.
ii) Silica NPs

Silica nanoparticles, according to studies, are very toxic to neuronal cells and have been associated with neurotoxicity and neurodegeneration. The SiO₂-NPs were administered via intravenous route in a mouse model and showed cognitive deficits with escalation of anxiety. Exposure to NPs was also found to manifest neurodegenerative features such as neuroinflammation, elevated phosphorylation of tau proteins, and impaired exocytosis. Mesoporous silica nanoparticles (MSNPs) are a subclass of silica nanoparticles that have remarkable properties, including a large surface area and enhanced drug loading and functionalization capabilities that are garnering interest.
iii) Cerium oxide NPs
Cerium oxide nanoparticles (CeONPs), better known as nanoceria, are endowed with antioxidant and radical-scavenging properties that promise therapeutic applications in treating neurological disorders. Small CeONPs of less than 5nm size can traverse the BBB. Reactive nitrogen species may be taken up and stored by brain cells for signaling reduction. Besides, they proffer protection against mitochondrial fragmentation. In addition, the neuroprotective effects of nano-ceria are postulated to condition advantages for a human AD model.
Future Perspectives
At present, therapeutic methods do not hold any hope for effective prevention of progression of NDs into their neurophysiological complexity and sophisticated pathways. Despite exhaustive research indulging in painstaking trial outcomes, the reality of therapy has yet again been found to be unsatisfactory, which demonstrates the disjunction between research and testing. In the past decades, there has been an attempt to use various drugs, biomolecules (such as proteins, peptides, and mAbs), and even phyto-constituents, around which treatment has been attempted to discover better modalities for using NDs. Gene therapy is being introduced as another strategy in treating NDs, which requires knowing the specific type of NDs along with the set of genes involved in the progression of the disorder. Using neuronal stem cells and developing therapeutic interventions aimed at modulating the differentiation process of NSCs so as to influence the rate of neurogenesis may be one future approach to address the multitude of neurological diseases.
Conclusion
While there are several active pharmaceutical agents that are present for treating NDs, most never make it across the BBB to elicit amazing results in clinical trials. Nanomedicine is a cutting-edge approach that is likely to obliterate the barriers associated with conventional medicine. Nano biomaterial-based targeting schemes are promising alternatives yet being explored to alleviate these hurdles since such methodologies allow modification of certain neural circuits confined to unique brain areas and hence bring about desired therapeutic effects within targeted brain regions.
References
1. Aridon P, et al. Protective role of heat shock proteins in Parkinson’s Disease. Neurodegener Dis. 2011;8(4):155–68.
2. Cano A, et al. Current advances in the development of novel polymeric nanoparticles for the treatment of neurodegenerative diseases. Nanomedicine. 2020;15(12):1239–61.
3. Dowding JM, et al. Cerium oxide nanoparticles protect against Aβ-induced mitochondrial
fragmentation and neuronal cell death. Cell Death Differ. 2014;21(10):1622–32.
4. Gordillo-Galeano A, Mora-Huertas CE. Solid lipid nanoparticles and nanostructured structured lipid
carriers: a review emphasizing on particle structure and drug release. Eur J Pharm Biopharm. 2018;133:285–308.
5. Khan MM, et al. Statistical optimization of co-loaded rifampicin and pentami dine polymeric
nanoparticles for the treatment of cutaneous Leishmaniasis. J Drug Deliv Sci Technol.
2023;79:104005.
6. Liu R, et al. Advances of nanoparticles as drug delivery systems for Disease diagnosis and treatment. Chin Chem Lett. 2023;34(2):107518.
7. Nguyen TT, et al. Nanotechnology-based drug delivery for central nervous system disorders. Volume 143. Biomedicine & Pharmacotherapy; 2021. p. 112117.
8. Patel V, Chavda V, Shah J. Nanotherapeutics in Neuropathologies: obstacles, challenges and recent advancements in CNS targeted Drug Delivery systems. Curr Neuropharmacol.
2021;19(5):693–710.
9. Raghav M et al. Nose-to-brain drug delivery: challenges and progress towards brain targeting in the treatment of neurological disorders. J Drug Deliv Sci Technol, 2023: p. 104756.
10. Singh K. Dendrimer-drug conjugates in drug delivery and targeting. Pharm Nanatechnol.
2015;3(4):239–60.