Aging is the natural transformation to the body over the course of the human lifespan. It occurs from the interaction of loss of youthful rejuvenating qualities and acquisition of harmful traits, keeping the balance between these factors towards a degenerative process over time. Aging has both quantitative and qualitative impacts upon life. The longevity is a measure of time lived while the quality, or health span, is the length of life during which one can live to full capacity without diseases. The impact of aging does not spare any organ system, and older age is a firmly established risk factor for diseases. Alterations in biological age because of environmental influences or inherent characteristics, including exposure to chemicals, reproductive function, metabolic alterations, and elements affecting epigenetic expression are believed to contribute to the noticed difference in individual’s lifespan. In spite of decades of observations and studies, aging process has not been effectively modified by human intervention and yet the anti-aging therapies and cosmetics market is estimated to grow by 50% over the next 5 years. Despite of this tremendous interest, the area remains in its infancy and fueled by grand promises, with minimal progress made towards halting this process.
Mechanisms of Aging
The driving forces behind the aging phenotype and biological age are multifactorial, complex, and mechanistically linked. It was initially suggested that this process started through the accumulation of somatic mutations in cells, and different frame works of aging have emphasized cellular and genetic trends observed during the aging process. However, there are still large gaps in this hypothesis, and DNA damage as a unifying theory remains to be established. It is increasingly apparent that aging phenotype is the consequence of many changes to several molecular pathways that control homeostatic processes. In 2013, Lopez-Otin et al. introduced a new paradigm for perceiving aging mechanisms, and outlined nine critical changes that occur during normal aging and whose manipulation can affect the aging process. Several of these mechanisms have been targeted by anti-aging therapies mechanistically. These mechanisms are, however, extremely interconnected and interventions designed to target one mechanism are often studied within a narrow scope, while the aging phenotype is uniquely expressed through various mechanisms at every biological scale in an organism. Activation of a single aging mechanism does not necessarily provide an accelerated aging phenotype. For instance, individual mutations at the genetic level in progeroid animal models lead to skewed patterns of health but overall normal lifespan. Similarly, intervention on as specific mechanism would not necessarily abate aging. Three mechanisms of aging which are extremely interdependent and are new targets for therapeutic interventions are: (a) Cellular Senescence, (b) production of Reactive Oxygen Species (ROS), and (c) Stem Cell Depletion.
Cellular Senescence
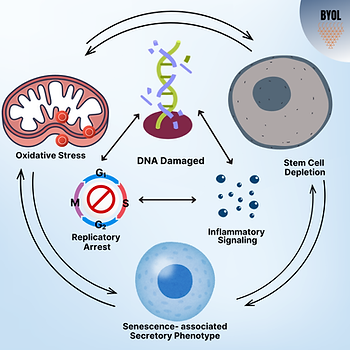
In cellular senescence, following serial replication, replicatory function is inhibited but the cell is alive and resistant to apoptosis. Reprogrammed quiescent and functional cellular tissue elements convert to pathologic phenotypes that are assumed to contribute to the generation of aging as a result of adverse cellular signaling from the reprogrammed cells. Various conditions, such as exposure to protein aggregates, advanced glycosylation end products, damage to DNA, ROS mediators, and inflammatory cytokines, can trigger this cell fate. Pro-inflammatory signaling through interleukins and cytokines may also further amplify NF-κB, p38MAPK, and associated signaling pathways that are thought to play a role in the development of the senescent phenotype. In response to DNA damage, p53/p21WAF1/CIP1 is induced that leads to cell cycle arrest and the replicative-inert senescent trait. Senescent cells also affect tissue homeostasis by the Senescence‐Associated Secretory Phenotype (SASP), which is linked to most age-related disorders, most significantly cancer, and telomeric shortening. With this phenotype, senescent cells overproduce inflammatory cytokines, chemokines, growth factors, matrix metallo-proteinases, and nitric oxide. Additionally, senescent cells overproduce profibrotic mediators, stem cell–dysregulating factors, and other damaging biological mediators. Further, SASP cells are capable of inducing ROS damage by cytokine signaling and direct stem cell dysregulation, leading to the activation of other aging hallmark features.
Oxidative Stress
Reactive Oxygen Species (ROS) signaling affects the aging phenotype. It has been observed that the amplitude and duration of ROS expression can produce contrasting effects. For instance, knockdown of NAPDH oxidase results in chronic, low levels of ROS expression, which in turn induce compensatory mechanisms and enhance longevity. However, sustained expression of high concentrations of ROS is a known risk factor linked with the aging phenotype, partly due to the progressive damage over time from loss of intrinsic antioxidative processes in conjunction with enhanced pro-oxidative stressors. Expression of ROS is triggered by numerous sources, such as mitochondrial pathology, inflammatory pathways, and extrinsic stressors like UV radiation. ROS is a polyfunctional signaling moiety, which affects cellular senescent pathways, DNA damage response and repair, and metabolism. These signaling pathways affect stem cell aging and the gain of the SASP. Overproduction of ROS has been associated with numerous diseases including cardiovascular disease, diabetes, and cancer. This central role in various cellular processes and diseases has rendered targeting ROS accumulation a promising field for modeling aging and for anti-aging interventions.
Stem Cell Depletion
Impaired replicative capacity of tissue-specific stem cells has been suggested to play a role in development of the aging phenotype. Stem cells reside within niches in tissues mainly for maintenance of normal tissue function, and with stimulatory signals, the stem cells change from quiescent to proliferative state to replace lost or damaged cells. Their long-term presence increases the probability of damage over time, which results in ineffective functionality and replication, a blunted response to tissue injury, and decreased healing and maintenance of tissues in aged organisms. Dysregulation of the microenvironment for stem cell populations triggers loss of this function. Extrinsic factors like inflammatory signaling, ROS-mediated injury, and metabolic disturbances can cause the accumulation of DNA damage, and this may result in senescence and replicatory arrest of stem cells. Metabolic derrangements through insulin-like growth factor 1 (IGF-1), mTOR, Wnt, and TGF-β affect stem cell depletion during aging and may result from metabolic syndromes and lipodystrophy. Studies have revealed that the Hematopoietic stem cells in older mice had decreased proliferation as a result of enhanced cytokine signaling and loss of the supportive stromal cell population. A higher failure rate of stem cell transplantations and engraftments in older individuals is due to this aggressive microenvironment. Modeling the effects of the young versus old microenvironment, and deriving methods to restore the young microenvironment, has gained interest as a rejuvenation strategy.
Therapeutic Strategies
Anti-aging medications have become the focus of heightened interest in the biotechnology industry, with numerous pharmaceutical and biotech firms putting such development on the top list of their research and development priorities. Anti-aging therapies concentrate on prevention of damage over a period of time (Ge+roprotection), restoration of youthfulness with the capability to replace tissues after injury (Rejuvenation and Regeneration). Most of these anti-aging approaches are based on dampening one of the hallmarks of aging or focusing on a particular age-related disease. We present here four promising areas of interest in the research of anti-aging interventions and comment on the evidence for their application and their limitations.
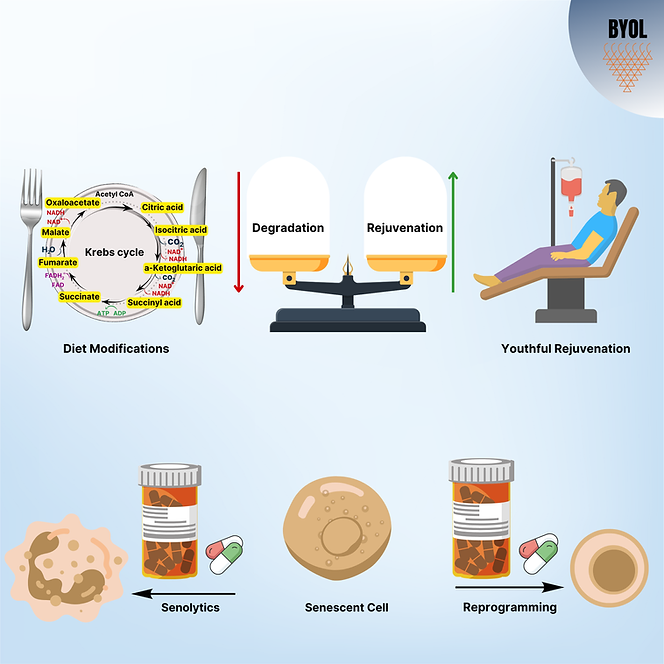
Geroprotective Strategies
These interventions aim to prevent the degradation rate with time by reducing exposure to pro-aging inducers.
Dietary modifications
Dietary changes and consequential metabolic alterations are promising as an easily accessible means of mitigating aging. Metabolic pathway modifications, including transition from anabolic to catabolic state, have been suspected in the process of aging that culminates in cell stress and ROS signal production and release. Alteration in pathways like IGF signaling, GH signaling, and mTOR pathways has been reported to lengthen lifespan in numerous non-vertebrate models. Analysis of human centenarian tissues has discovered that particular genetic loci like TOMM40/APOE and FOXO3a, are associated with extended longevity. Thus, different strategies modulating metabolic processes are becoming targets as an anti-aging intervention.
Studies have revealed that caloric restriction is able to prolong lifespan. Caloric restriction is the reduction of caloric consumption below normal levels while keeping adequate nutritional content. Caloric restriction induces changes in DNA methylation, post-translational histone modifications, and other epigenetic modifications. A meta-review of caloric restriction intervention among animal and human trials have revealed that caloric restriction enhanced the life span of yeasts, flies, worms, fish, and rodents. In humans, a 2-year clinical trial of caloric restriction reported no alteration in the circulating levels of IGF-1, GH, or other caloric restriction-sensitive markers but did report favorable alterations in body weight, glucoregulatory function, and cardiovascular disease markers. Eventually, caloric restriction has been estimated to increase the lifespan of human beings by 1–5 years. Emerging evidence proposes that intermittent fasting, or a reorganization of the pattern of caloric intake as opposed to the amount of calories consumed, might trigger positive benefits akin to caloric restriction. Fasting durations activate metabolic changes such as those associated with caloric restriction, which has the positive effects to avert diseases and deterioration. Antioxidative diets have also become popular in an attempt to moderate the pace of aging, and numerous commercial items now employ the antioxidative descriptor as a sales tactic. There have been various studies examining mutations in Superoxide Dismutase and Catalase, two antioxidant enzymes, where the mutations were found to produce an increase in lifespan in Drosophila.
Collectively, exercise and diet are the most uncomplicated way to reduce the effects of aging and other disease processes. Although well studied and extremely promising, dietary changes are challenging to adhere to and need continued effort and dedication. Thus, other approaches involving pharmaceutical mimetics are being contemplated. Numerous commonly used drugs, including Aspirin, Metformin, Rapamycin, and Resveratrol, have shown metabolic modulation.
Senolytics
Baker et al. in 2011 showed that clearance of senescent cells in a transgenic mouse model favored longevity and a youthful phenotype. There are few drugs classified directly as senolytics including Dasatinib, Quercetin, Navitoclax and Fisetin. All of these have presented favorable findings in preclinical models to inhibit senescent cells, thus inducing the retardation and prevention of age- and senescence-related disease. One of the most commonly prescribed drugs for type II diabetes, Metformin, has been reported to lower the rate of DNA damage, cellular senescence, and mitochondrial oxidation while increasing rates of autophagy of pathogenic cells through a broad range of potential mechanisms. Metformin is also being researched in FDA-approved clinical trials as a disease-prevention approach to age-related illness. Other well-established therapeutics targeting the mTOR pathway, such as Rapamycin or Sirolimus, have demonstrated senolytic properties and are currently under investigation. Recently a cell-based strategy involving senolytic CAR T cells prolonged the life of mice with lung adenocarcinoma treated with a senescence-inducing drug combination, and this strategy normalized tissue homeostasis in mice where liver fibrosis had been induced.
Rejuvenation and Regeneration Strategies
Another strategy to fight aging is by restoration of youthful characteristics, or rejuvenation. These strategies take advantage of our knowledge of embryonic development and stem cell biology to eliminate markers of damage that have been accumulated with age.
Cellular reprogramming
In 2006, it was shown that fully differentiated cells from a patient could be converted into an embryonic state with pluripotent differentiative potential and indefinite self-renewal. By inducing four important transcription factors, OCT4, SOX2, KLF4, and C-MYC (OSKM factors), cells can be reverted to the state of embryos, and they are potentially capable of differentiating into any cell type or tissue of the three germ layers: ectoderm, mesoderm, and endoderm. The idea on which this tactic is based is that, during the reprogramming process, the cells revert back to an adult stage and thus will impart a youthful nature on the organism. The induction process reduces levels of the senescence markers p16INK4A and p21CIP1 and reboots the epigenetic clock of DNA methylation. This might be a more desirable approach than the direct targeting of methylation sites, as there are simply so many age-related sites of DNA methylation (∼500,000). This procedure seems to be mediated by a cytokine signal cascade intersecting through NF-κB and IL-6 signaling-mediated. Cellular reprogramming, therefore, has promising therapeutic applications in the treatment of age-related diseases, even though its induction is not without risk. Prolonged expression of OSKM genes has yielded teratomas and cancers derived from reprogramming. However, Cyclical OSKM gene expression seems to have overcome this shortcoming, and there are no reports of cancer in experiments employing this method.
Studies have prompted companies to utilize reprogramming as a human rejuvenation process. Numerous examples of this process have been shown in vitro, but there are no human clinical trials based on cellular reprogramming due to numerous obstacles to its clinical use, thus challenging its future use. The models on which it was shown to be effective are genetically modified organisms with promoter-engineered expression of OSKM factors to ectopically express the factors, which cannot be translated into humans.
Modulating the stem cell niche
Rejuvenation and regeneration have been reported in aged parabionts following exposure to young serum, leading to histological organ-level changes throughout tissues in all three primordial germ layers. The results of these experiments have led to the investigation of the components of young blood as a rejuvenation therapy. Parabiosis research indicates that young blood could have rejuvenation-facilitating factors that decrease with age and that dilution of old serum reduces the accumulation of negative factors. Sinha et al. and Katsimpardi et al. have reported that GDF11 was high in young serum responsible for muscle regeneration and olfactory neurogenesis. Such results from parabiosis experiments are in need of further study before application in human beings.
Conclusions
The area of anti-aging therapeutics is an emerging field and at present, it is marked by new approaches developing to reverse the aging phenotype followed by the lack of interest as a result of disappointing outcomes. In order to move the field towards substantive and significant work, some reform has to occur. In particular, there has to be standardization of methodology and baseline analysis for all the therapeutic candidates so that they may be screened to identify interventions that have the best potential to treat aging.
References
- Moskalev A A, Shaposhnikov M V, Plyusnina E N, Zhavoronkov A, Budovsky A, et al. 2013. The role of DNA damage and repair in aging through the prism of Koch-likecriteria. Ageing Res. Rev.12(2):661–84
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, et al. 2011. Clearance of p16Ink4apositive senescent cells delays ageing-associated disorders.Nature 479(7372):232–36
- Sinha M, Jang YC, Oh J, Khong D, Wu EY, et al. 2014. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle.Science 344(6184):649–52
- KatsimpardiL,LittermanNK,ScheinPA,MillerCM,LoffredoFS,etal.2014.Vascularandneurogenic rejuvenation of the aging mouse brain by young systemic factors.Science 344(6184):630–34
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging.
- Kirkland J, Tchkonia T. 2020. Senolytic drugs: from discovery to translation. J. Intern. Med. 288 (5):518– 36 ell 153(6):1194–217
- Lopes-Paciencia S, Saint-Germain E, Rowell M-C, Ruiz AF, Kalegari P, et al. 2019. The senescenceassociated secretory phenotype and its regulation.Cytokine 117:15–22
- LehallierB,ShokhirevMN,Wyss-CorayT,JohnsonAA.2020.Dataminingofhumanplasmaproteins generates a multitude of highly predictive aging clocks that reflect different aspects of aging.Aging Cell 19(11):e13256