Embryo transfer is a process involving removal of one or more embryos from a donor female’s reproductive tract and placing them into one or more recipient females. Embryos can also be created in the laboratory through in vitro fertilization or somatic cell cloning methods. The actual transfer process of an embryo involves some or all of the following:
- Super-ovulation and insemination of donors.
- Embryo collection.
- Isolation, assessment, and short-term storage of embryos.
- Micromanipulation and genetic analysis of embryos.
- Embryo freezing.
- Embryo transfer.
Now-a-days, embryo transfer technology (ETT) is regarded as the main method that is highly essential for obtaining success in most assisted reproductive technologies, particularly in in-vitro fertilization and animal cloning. Besides natural and artificial insemination, higher pregnancy rates have been obtained through the assistance of embryo transfer methods. It is estimated that upcoming scenario of increased economic values of animals and livestock products will end up in a drastic use of embryo transfer technology. But for the attainment of an improved success rate of embryo transfer, there is a great challenge ahead and a need for correct theoretical and technical know-how in order to gain a higher success rate in embryo transfer technology.
Primary Role of Embryo Transfer Technology
- Better utilization of female reproductive performance as in producing more off-springs from worthy donors through the use of MOET (Multiple Ovulation Embryo Transfer Technology).
- Embryos are utilized for conservation of desirable genetic materials and to transfer genes (Transgenics).
- The embryo transfer technique can also produce twin embryos, manipulate embryos and develop new breeding concepts as genotypes, shorten generation intervals, test for desirable genes in short time, etc.
- Embryo transfer can also be advantageous to facilitate import and export of valuable genetic materials and to study embryology, embryo-genomics, early pregnancies, as well as preservation of endangered species.
Steps in Embryo Transfer Technology
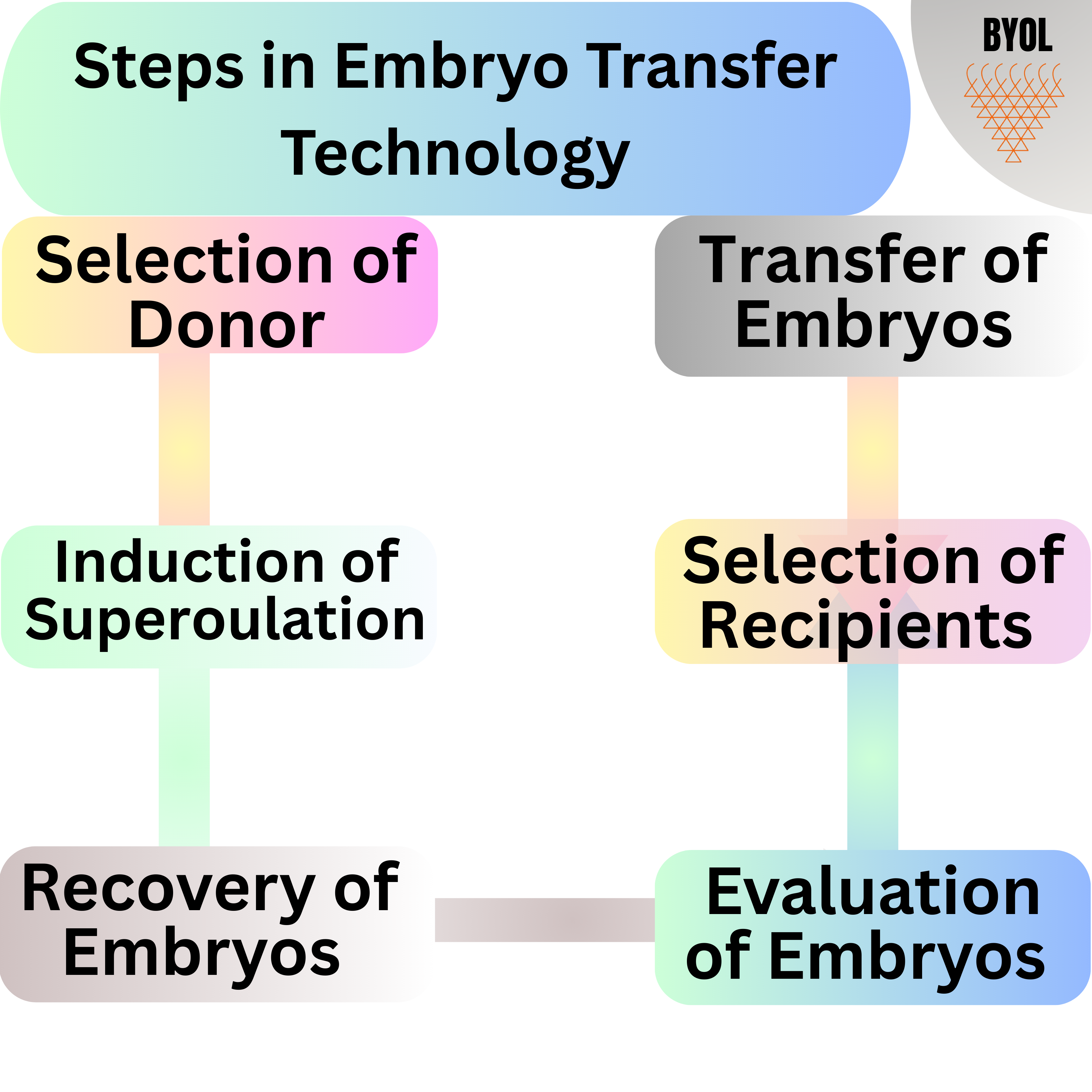
1 Selection of Donor
Following considerations should be made for choosing a donor animal:
- Genetic excellence of the donor.
- Breed purity under which the donor animal is being chosen.
- Normal physiology and health status.
- Normal reproductive status, age, and economic value of the potential offspring.
For dairy cows, the highest cow index value is the best measure of excellent genetic potential. Only cows with high cow index and popular pedigree with a depth of good breeding and production should be employed as donors. Cows should be thrifty and disease-free, should regularly cycle, and should have the history of regular calving. Typically, older cows which have produced well, have had several superior offsprings and have classified as high are chosen as donors, but dairy producers should not overlook heifers with a high cow index.
2. Induction of Superovulation
- The concept of superovulation is to introduce more ovulations than the regular rate by administering a gonadotropin stimulus, followed by regulation of luteolysis, simultaneous ovulation, high fertilization and early embryonic development rates.
- The preparations to induce superovulation are Pregnant Mare`s Serum Gonadotropin (PMSG or eCG), Follicle-Stimulating Hormone (FSH), and Human Menopausal Gonadotropin (hMG).
- The PMSG is a glycoprotein that produces both Follicle-Stimulating Hormone (FSH) and Luteinizing hormone (LH) biological effects. Because of its carbohydrate side chains and sialic acid, PMSG has an exceedingly long half-life of approximately 5 days. A luteolytic dose of Prostaglandins (PG) is given 2 to 3 days after PMSG treatment, and the donor is supposed to exhibit heat signs 2 days following PG injection. The advantage of utilizing PMSG is its low-cost availability and ovulation can be triggered with its single dose. The main disadvantage of PMSG treatment is long half-life of PMSG inducing extra follicular growth with a resultant increased estradiol secretion that persists during post-ovulatory period affecting early embryonic development.
- FSH treatment is reported to have enhanced and more consistent ovulatory reaction. The half-life of FSH is about 5 hours. For achieving a best response, the ratio of FSH to LH of 5:1 should be administered.
- The hMG is a protein purified from urine of menopausal women having both FSH and LH activities. Superovulation can be properly induced by giving only a single dose of 450 to 600 IU of hMG.
3. Recovery of Embryos
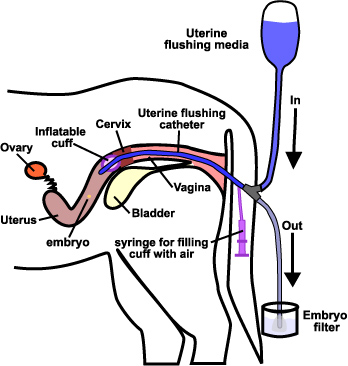
- In cattle and buffalo, embryos are harvested by nonsurgical techniques in which a catheter of special design is inserted through the cervix into uterine cornua and embryos are flushed out with 250-300 ml flushing medium.
- The rate of embryo recovery varies with the operator who is doing the flushing, position of the embryos within the uterus, time of recovery, super-ovulatory response, and embryo viability, etc.
- It has been observed that from a single flush an average of 4-6 transferable embryos are obtained. Further, flushing slightly beyond the uterine bifurcation has provided better embryo recovery compared to flushing at the tip of the uterus.
- In sheep, goat, and pigs, embryo recovery is done by surgical techniques. Donor’s abdomen is opened via a midline incision, and embryos are recovered by flushing the oviduct and/or the uterine horns.
4. Evaluation of Embryos
The two primary approaches for evaluating embryo viability are morphological and staining methods.
Morphological Method
This involves microscopic examination at different magnifications to observe the embryo’s developmental stages. Key criteria used in morphological assessment include:
- Shape and color of the embryo.
- Number of blastomeres.
- Degree of cell compactness.
- Size of the perivitelline space.
- Number and size of vesicles.
- Condition of the zona pellucid.
Staining Method
This technique employs vital staining and fluorescence methods to assess cellular viability and structure. This method helps distinguish live cells from dead ones and provides insight into the metabolic state of the embryo.
5. Selection of Recipients
The criteria for selecting recipient animals include the health condition of the animal, reproductive status, compatibility to the donor with respect to the size of the foetus and consistent oestrus synchronization. The oestrus synchronization of donors and recipients should occur within 24 hours, otherwise, there will be a significant reduction in pregnancy rates because the highest conception rate is achieved when an embryo is transplanted into a uterine environment closely resembling the one to which it originated.
Methods of Synchronization
- Natural Estrus: Selecting recipient females that are naturally in estrus at the same time as the donor can achieve synchronization.
- Hormonal Synchronization: Prostaglandin F2α (PGF2α) or progesterone-releasing devices can be used to manipulate the estrous cycle of recipient females, bringing them into estrus at a predetermined time.
- GnRH: Gonadotropin-releasing hormone (GnRH) can also be used to synchronize follicular wave emergence and ovulation in both donors and recipients.
6. Transfer of Embryos
For achieving success in embryo transfer, a number of factors are important which includes:
- Good quality embryos.
- Embryo should be transferred into uterine horn bearing corpus luteum.
- Recipients should be healthy and free from any reproductive disorder.
- Good expertise in handling and transfer of embryos.
Non-surgical method
- The recipient animal is administered a light epidural anesthesia between the lumbar vertebrae to help diminish rectal contractions.
- The embryo to be transferred is taken up into a 0.25 ml straw then transferred into the AI gun.
- Left hand is placed in the rectum and the cervix is palpated and grasped firmly.
- The insemination gun is carefully passed through the cervix and into the uterus corresponding to the ovary bearing corpus luteum.
- The embryo should be disposed as deep into the uterine horn as possible without using force. If twins are required, embryos should be placed in both uterine horns of the recipient having corpus luteum,
Surgical method
- The recipient is prepared for surgery by shaving an area in front of the hip joint. A local anaesthetic is injected at the shaved area. The area is scrubbed with alcohol and a 2 inch incision is made with a scalpel.
- The uterus and the ovaries are brought near the incision site by grasping the uterus with the fingers. A small incision is made in the exposed uterine horn with a blunt needle.
- The embryo is drawn into a 0.25 ml straw attached to a small syringe and deposited into the uterus. The incision is closed with a few stitches and antibiotic solution is applied into the stitch area to remove infection.
Cryopreservation of embryos
- Cryopreservation is a vital component of embryo transfer programs.
- Blastocysts with a multi-layered trophoblast are ideal for rapid freezing and thawing.
- Reliable protocols have been established, particularly for bovine and sheep embryos. These involve a one-step addition of 1.4 M glycerol as a cryoprotectant, 20 minutes of equilibration, packaging in 0.25 ml straws, slow cooling at 0.3 to 0.1 °C/min to –35 °C, followed by immersion in liquid nitrogen (–196 °C). The preserved embryos can be thawed and used when needed.
Conclusions
- Embryo Transfer Technology (ETT) has evolved into a cornerstone of assisted reproductive technologies (ART) due to its consistent success rates and its ability to enhance genetic progress in livestock. Over the years, what began as an experimental approach has gained widespread acceptance, supported by a strong track record of efficacy and safety. Its increasing application across veterinary science has reinforced its status as a mature and vital tool in the reproductive sciences.
- The industry’s growth is reflected not just in its expanding market value but also in the intensifying research dedicated to refining the process, minimizing risks, increasing efficiency, and expanding its application to a broader range of species and reproductive scenarios. As a result, ETT now commands significant interest from researchers and commercial breeders alike, underlining its pivotal role in shaping the future of reproductive technologies.
References
- Hasler JF. Forty years of embryo transfer in cattle: a review focusing on the journal Theriogenology, the growth of the industry in North America, and personal reminisces. Theriogenology. 2014; 81:152-69.
- Bo GA, Mapletoft RJ. Historical perspectives and recent research on superovulation in cattle. Theriogenology. 2014; 81:38-48.
- Bo GA, Mapletoft RJ. Strategies for donor and recipient selection, treatment, and management. In: Proceedings American Embryo Transfer Association, Champaign, IL, 2013, 16-24.
- Jahnke MJ, West JK, Youngs CR. Evaluation of in vivo derived embryos. In:Hopper RM, editor. Bovine reproduction. Hoboken (NJ): Wiley and Sons, 2014, 73348.
- Oetzel GR. The effect of nutrition on reproduction: applications for ET donors and recipients. In: Proceedings American Embryo Transfer Association, Champaign, IL; 2012, 50-9.