Brucellosis is a zoonotic disease of considerable importance to veterinary and public health, significantly affecting livestock output economically in many poor countries, including India. The OIE identifies it as the second most significant zoonotic disease globally, following rabies, with a yearly incidence of 500,000 cases worldwide. Brucellosis is recognised as one of the most prominent neglected zoonotic diseases globally. This illness is among the most catastrophic transboundary animal diseases and is a significant trade impediment. The disease impacts a broad spectrum of domestic and wild animals as main hosts, with humans serving as secondary hosts. Brucellosis was initially identified as a human disease on the island of Malta in the 19th and early 20th century.
Etiology
- Brucellosis was initially identified in 1887 by British surgeon David Bruce, who isolated Gram-negative coccobacilli from the spleens of five British soldiers who succumbed to fever in Malta, a condition referred to as Malta fever, prevalent among military personnel stationed on the island. The bacteria were designated Micrococcus melitensis, with “melitensis” originating from the Roman appellation for Malta, “Melita.” In 1897, Bernhard Bang discovered Bacillus abortus as the etiological agent of infectious abortion in cattle. Subsequently, in 1917, it was discovered that the aetiologies of the two diseases were identical, leading to the renaming of Brucella in tribute to Bruce.
- Currently, 12 species of the Brucella genus have been identified, of which six are recognized as harmful to both animals and humans. B. abortus preferentially infects bovines, B. melitensis sheep and goats, B. suis pigs, and B. canis dogs, whereas the other members comprise B. ovis and B. Neotomae. Brucellosis can be transmitted among cattle, swine, sheep, goats, and various other species, including dogs, horses, bison, reindeer, and camels.
- Brucella melitensis is responsible for the majority of global cases and exhibits a tendency for recurrence and chronicity. They are Gram-negative, somewhat acid-fast, aerobic, facultatively intracellular coccobacilli or short rods.
- Brucella species and their various biotypes are presently differentiated by differential tests that include serotyping, phage typing, dye sensitivity, CO2 demand, H2S generation, and metabolic characteristics.
Transmission and risk determinants
Animals
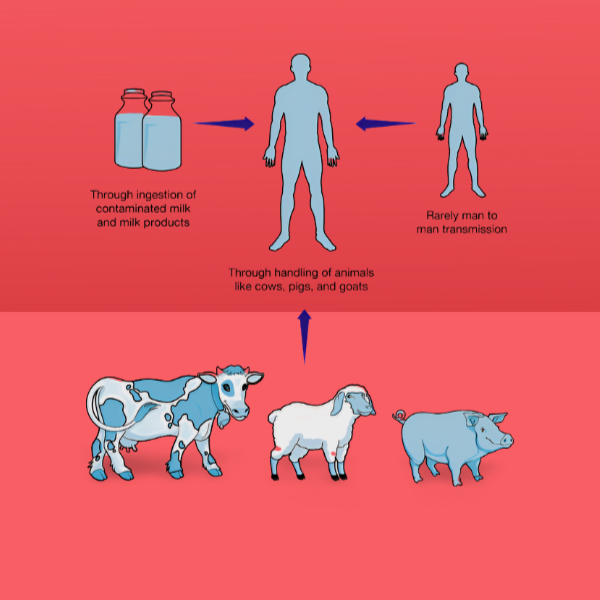
- The primary route of transmission in animals is ingestion however, transmission may also occur through inhalation of infected aerosols, conjunctival inoculation, skin contamination, and udder inoculation via contaminated milking cups.
- Aborted foetuses, along with foetal membranes and uterine fluids expelled following abortion or parturition, represent the primary reservoirs of infection.
- Animals acquire the infection by the consumption of contaminated feed and water, as well as by licking aborted materials, foetuses and the vaginas of infected animals.
- The disease may also be transferred vertically to calves and by infected milk; however, these transmission pathways are significantly less critical.
- Venereal transmission is infrequently implicated in the epidemiology of cattle brucellosis; nevertheless, artificial insemination with contaminated semen poses a possible infection risk.
Humans
- Humans serve as incidental hosts; yet, brucellosis remains a significant global public health issue. The prevalence of human brucellosis is influenced by factors such as husbandry practices, dietary patterns, milk processing procedures, dairy product handling, and environmental sanitation.
- Humans contract the virus from infected animals through eating (unpasteurized milk or dairy products), inhalation of aerosols, conjunctival exposure, or contact with infected animals and their products.
- Brucellosis mostly affects abattoir workers, veterinarians, laboratory technicians, hunters, farmers, and livestock producers because of their direct contact with sick animals and their tissues.
- Human-to-human transmission is infrequent; nonetheless, it can occur by blood transfusion, bone marrow transplantation, sexual contact, or congenital means.
- Veterinarians may contract the virus when assisting in the delivery of diseased livestock and by inadvertent exposure to vaccines.
Symptoms
Animals
- The condition in animals is referred to as enzootic abortion, epizootic abortion, infectious abortion, and Bang’s illness.
- It is a sub-acute or chronic condition that can impact several domestic and wild animal species.
- During the early stage of infection, the disease is asymptomatic (WHO, 2006). Brucellosis in animals diminishes output by inducing miscarriages, impairing fertility, and lowering milk production.
- The disease presents with late-term miscarriages, weak calves, stillbirths, and infertility, mostly characterised by placentitis, epididymitis, and orchitis, with the organisms excreted in uterine discharges and milk. Infected bulls may exhibit systemic indications of infection such as fever, anorexia, and lethargy.
- Brucellosis in pregnancy poses the possibility of spontaneous abortion or intrauterine transmission to the foetus.
Humans
- The illness in humans is referred to as undulant fever, Malta fever, and Mediterranean fever.
- In humans, brucellosis affects multiple organs and presents with a complex array of clinical manifestations, varying from nonspecific to severe symptoms, which facilitates its misdiagnosis as other diseases.
- Human brucellosis is divided into three categories based on disease progression:
- Acute brucellosis is marked by weakness, undulant fever, headaches, myalgia, a fine red rash, splenomegaly, hepatomegaly, and gastrointestinal symptoms. The acute phase may culminate in death, resolution, or progression to a sub-acute or chronic form.
- Sub-acute brucellosis presents with nearly all symptoms characteristic of the acute phase but in a milder form.
- Chronic brucellosis, where prolonged manifestations may include fatigue, undulant fever, arthritis, endocarditis, spondylitis, and alterations in personality. It is frequently misinterpreted as other febrile syndromes, including malaria and typhoid fever, leading to inappropriate treatments and underreporting.
Diagnosis
The diagnosis of brucellosis requires an assessment of medical history, clinical evaluation, routine laboratory and radiologic testing, along with culture, serology, or polymerase chain reaction (PCR) assay.
- The gold standard specific test for diagnosis of Brucella spp. is bacterial isolation but it is time-consuming and necessitates biosafety level 3 and skilled staff.
- Molecular methods are efficient and expedient for diagnosing Brucella spp. from serum, blood, pus, and tissue. Currently, real-time quantitative PCR (qPCR) is regarded as a highly reliable method for detecting Brucella contamination in milk and may facilitate the differentiation between virulent strains and those arising from vaccination.
- The standard and most accessible serological assays for identifying specific antibodies against Brucella antigens in milk and serum include the Milk Ring Test (MRT), Rose Bengal Test (RBT), Standard Tube Agglutination Test (SAT), Coombs Test, and Complement Fixation Test.
- The Rose Bengal test serves as a screening tool, with positive samples verified by the SAT. The Rose Bengal plate test exhibits a sensitivity exceeding 99%.
- The SAT continues to be the most well recognised diagnostic assessment globally. The SAT quantifies the total amount of agglutinating antibodies (IgM and IgG), whereas the specific quantity of IgG is assessed using 2-mercaptoethanol (2ME). SAT levels exceeding 1:160 are deemed diagnostic when accompanied by a corresponding clinical manifestation. In endemic regions, a cutoff titre of 1:320 may enhance the specificity of the test.
- The complement fixation test (CFT) is frequently employed for diagnosing brucellosis in cattle, sheep, and goats. This test primarily detects IgG1 antibodies; nevertheless, it is very intricate and cannot distinguish between infected and vaccinated animals.
- The enzyme-linked immunosorbent assay (ELISA) that quantifies IgG, IgM, and IgA antibodies has great sensitivity and facilitates improved interpretation of clinical scenarios. Nonetheless, the specificity of ELISA is inferior to that of agglutination assays.
- The Coomb’s test is the most appropriate and sensitive method for confirmation in relapsing individuals with ongoing disease; nonetheless, it is intricate and requires skill.
- The lateral flow assay (LFA), a streamlined variant of ELISA, possesses significant potential as a quick point-of-care diagnostic tool. This test exhibits elevated sensitivity and specificity. This is a swift and straightforward diagnostic assay for the confirmation of brucellosis in an endemic region.
Therapeutic Intervention
- Despite the implementation of the WHO’s antibiotic regimen recommendation (1986), which includes Doxycycline 100 mg orally twice daily for 6 weeks in conjunction with oral Rifampicin 600 to 900 mg daily for 6 weeks or intramuscular streptomycin 1g daily for 2-3 weeks, the incidence of treatment failure and relapse in brucellosis has risen by 5-15%.
- The preferred therapy regimen for uncomplicated brucellosis is streptomycin for 2 to 3 weeks in conjunction with doxycycline for 8 weeks, or gentamicin for 5 to 7 days alongside doxycycline for 8 weeks.
- Second-line medications, such as quinolones or trimethoprim-sulfamethoxazole, may be offered to patients experiencing treatment failure or recurrent relapses.
- Treatment intervention for patients with a complex condition necessitates a meticulous assessment of the patient and a comprehensive therapeutic strategy. Patients diagnosed with spondylitis may benefit from the inclusion of a quinolone in the initial treatment regimen for an extended duration.
Prevention and Management

- The prevention and control of brucellosis in humans mostly rely on effective management of the illness in livestock. In 1998, the WHO advocated for standardized techniques to eradicate animal brucellosis. The strategies and programs encompass:
(1) Prevention of disease spread among animals and the monitoring of brucellosis-free herds and regions.
(2) Identification of infected animals through diagnostic testing and their subsequent elimination via slaughter programs to establish brucellosis-free herds and zones.
(3) Implementation of extensive vaccination programs to reduce disease prevalence.
- In endemic regions, pasteurization of milk and dairy products is seen as a crucial safety measure. Consumption of unpasteurized milk and dairy products, as well as raw or undercooked animal products (including bone marrow), should be avoided.
- Occupational exposure to Brucella can be mitigated through proper hygiene practices and the utilization of protective gear and equipment.
- Implementing safety measures is crucial to avert skin contamination, inhalation, or inadvertent ingestion of organisms during childbirth assistance, necropsy procedures, or animal slaughtering.
- The management of an aborted foetus, together with its membranes and fluids, necessitates certain precautions.
Enhanced veterinary services and public health education may significantly contribute to disease control. Some governments have accomplished these objectives through vaccination, a test-and-slaughter policy, and stringent regulation of animal transportation.
- Vaccination has significantly aided in the prevention and elimination of brucellosis worldwide. Vaccination is crucial for enhancing animal health and serves as a significant measure to mitigate the risk of severe illness and incapacity. Vaccination for brucellosis is implemented in nations with an incidence exceeding 5%, especially in developing countries, due to its cost-effectiveness and general acceptance among farmers.
- The presently available vaccinations for bovine and caprine brucellosis comprise live attenuated organisms. Cattle vaccines comprise either the smooth strain B. Abortus S-19 or the rough strain RB-51, whereas the caprine vaccine comprises the attenuated B. melitensis vaccine strain Rev-1. These live attenuated vaccines demonstrated efficacy in reducing abortion and the transmission of brucellosis, although had limited effectiveness in preventing infection or seroconversion.
- Educational initiatives aimed at at-risk populations, coupled with rigorously enforced hygiene measures, regulations, and inspections, should be instituted to diminish the prevalence of this illness in brucellosis-endemic areas.
Conclusion
Brucellosis is a zoonotic disease endemic in low, middle, and high-income nations, resulting in significant losses to the livestock sector. It imposes a considerable burden on human healthcare systems and constrains the economic potential of people, communities, and nations. The execution of public policy aimed at alleviating the socioeconomic impacts of brucellosis in both human and animal populations is urgently required. The interdisciplinary “One Health” initiative signifies the necessity for collaboration among veterinary, medical, public health, cultural, economic, and social professionals to mitigate the burden of brucellosis.
References
- Alsaif, M., Dabelah, K., Girim, H., Featherstone, R., and Robinson, J.L. 2018. Congenital Brucellosis: A Systematic Review of the Literature. Vector Borne Zoonotic Dis. 18(8): 393403
- Avila-Calderon, E.D., Lopez-Merino, A., Sriranganathan, N., Boyle, S.M. and Contreras-Rodriguez, A. 2016. A history of the development of Brucella vaccines. Biomedical Research Institute. 2013743509 [Online] Available from: http://dx.doi.org/10/1155/2013/743509
- Bakri, F.G., Hamzah, M., AlQadiri. and Adwan, M.H. 2018. The Highest Cited Papers in Brucellosis: Identification Using Two Databases and Review of the Papers‟ Major Findings. Bio Medical Research International. 10 Blasco.
- Dadar, M., Shahali, Y., Adrian, M., and Whatmore. 2019. Human brucellosis caused by raw dairy products: A review on the occurrence, major risk factors, and prevention. International Journal of Food Microbiology. 292: 39-47
- Giambartolomei, G.H. and Delpino, M.V. 2019. Immunopathogenesis of Hepatic Brucellosis. Frontiers in Cellular and Infection Microbiology. 9 (423): 1-9.
- Kumar, A., Gupta, V.K., Verma, A.K., Yadav, S.K. and Rahal, A. 2016. Vaccines for caprine brucellosis: status and perspective. International Journal of Vaccines & Vaccination. 2(3): 00030; http://dx.doi.org/10.15406/ ijvv.2016.02.00030.
- World Health Organization (WHO). 2006. Brucellosis in humans and animals. 188.